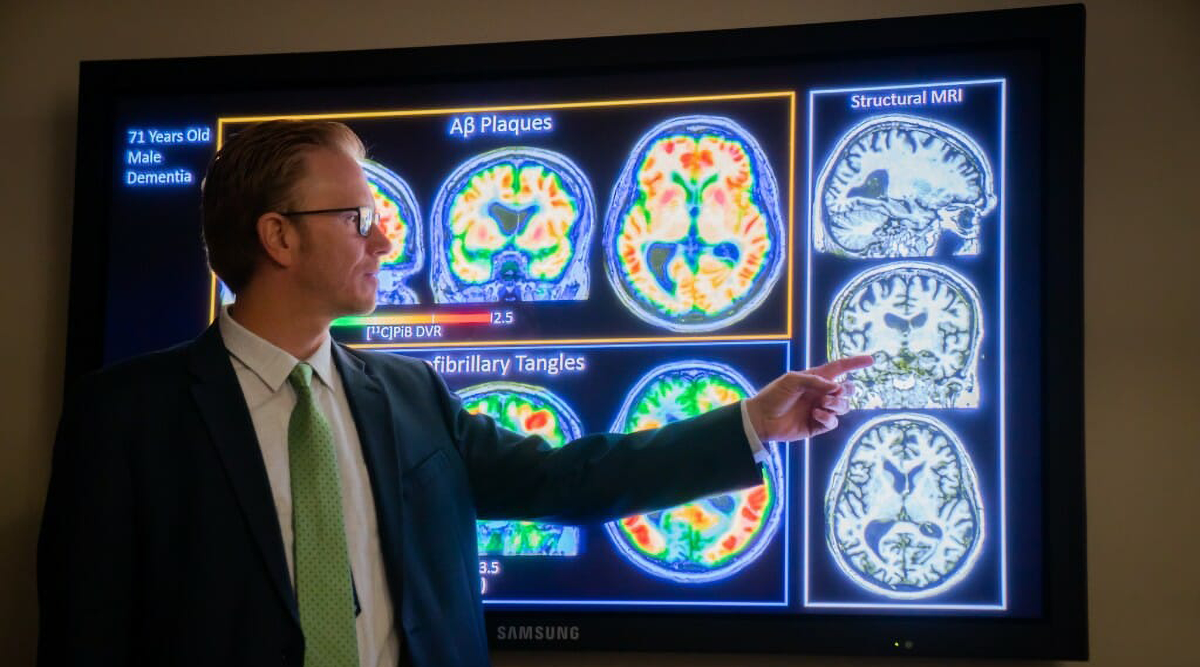
Seizures and memory problems in epilepsy may have a common cause
Damage to a part of the brain that regulates hyperactivity can contribute to both memory problems and seizures in the most common form of epilepsy, according to research at the University of Wisconsin–Madison....